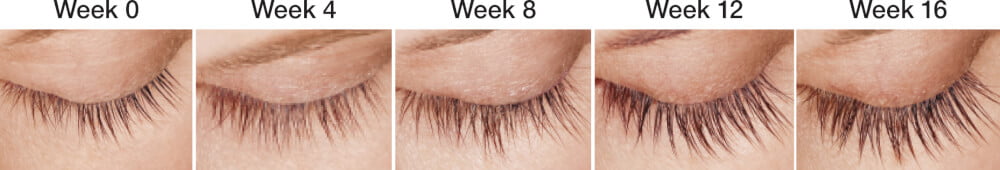
Top Trend — Thick Lashes.
The appearance of the eyes is central to the perception of beauty. Long, thick eyelashes and full eyebrows are widely regarded as desirable attributes of the eyes. Through the natural aging process, eyebrows and eyelashes frequently become thinner and lighter in color, known as hypotrichosis or alopecia. Currently, Latisse is the only FDA-approved product for the treatment of eyelash hypotrichosis.
What is Latisse?
Latisse is the brand name for bimatoprost ophthalmic solution 0.03%. It is a prostaglandin analog that was originally FDA approved in 2001 to treat elevated intraocular pressure in patients with open-angle glaucoma. The side effect of eyelash growth was noted, and it led to further study and FDA approval for treating thin eyelashes in 2008.
Latisse increases hair growth by increasing the number of hair follicles in the hair cycle’s growth phase. It increases thickness by increasing the size of the hair bulb. And, it amplifies lash darkness by increasing hair follicle production of melanin.
Does Latisse Really Work?
The effectiveness of Latisse was reported in a 2014 study that involved 585 patients from 16 different treatment centers. These patients had on average 19 months of Latisse use. Researchers found that 92% of the subjects were satisfied or very satisfied with the long-term use of Latisse for their eyelashes. Moreover, nearly 75% of participants had shifted to a maintenance regimen of only 3 treatments per week and saw sustained results.
No serious adverse events were reported in this study. Twenty-eight percent of patients did report a mild adverse event, including eye itching, eyelid redness, and eye irritation. However, less than 2% of patients discontinued Latisse due to these effects.
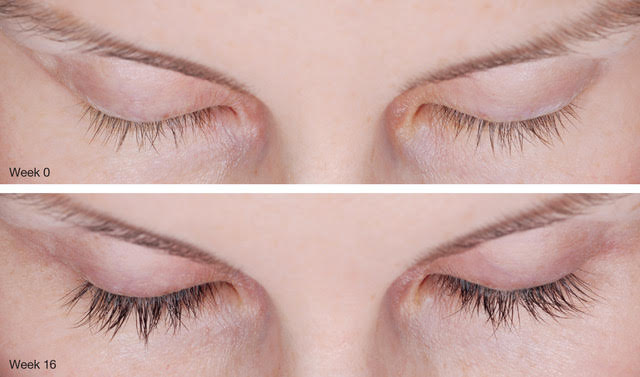
Long-term use of Latisse appears safe and cost-effective.
Is Latisse Safe For Blue-Eyed Individuals?
Yes! Although changes in the iris’ color (colored portion of the eye) are listed as a potential side effect, this is based on the research of glaucoma treatments in which bimatoprost drops were applied directly on the eye at 20-40 times higher doses.
This side effect was not observed in the previously mentioned study of 585 patients. Another study in 2017 looked at this question specifically. Fifty women who used Latisse over an average of 4 years (48% of whom had blue or green eyes) were evaluated for pigmentary change to the iris. No changes were seen in any patients.
Can I Use Latisse On My Eyebrows?
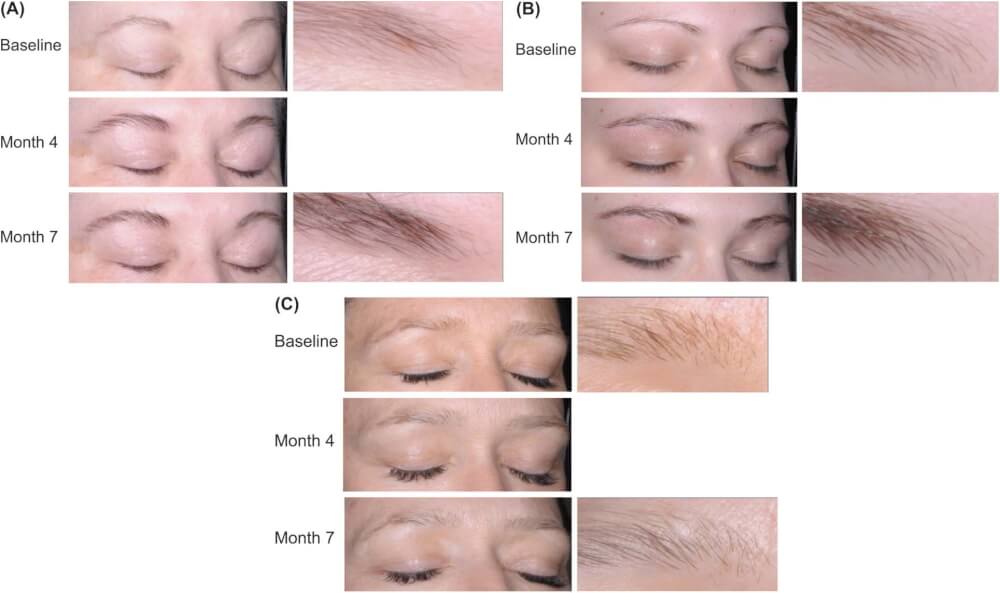
Yes! Given the success of Latisse in improving eyelashes, researchers have examined the safety and effectiveness of Latisse to stimulate eyebrow hair growth. An industry-supported study in 2016 that included 357 subjects found that about 80% of participants using Latisse showed at least a 1-grade improvement on the 4-point Global Eyebrow Assessment scale (GEBA). There was also a significant improvement in eyebrow darkness and fullness in the treatment groups compared with placebo.
Adverse events related to treatment were uncommon. Unintended hair growth in other facial areas, skin hyperpigmentation, and iris pigmentary changes were not observed.
Examples of Global eyebrow assessment scale (GEBA) changes over time representative of the three treatment groups. One subject receiving bimatoprost twice daily (A) and one receiving once daily (B) had GEBA grades of 2 (sparse) at baseline, 3 (full) at Month 4, and 4 (very full) at Month 7. The subject receiving placebo (C) had a GEBA grade of 1 (very sparse) at baseline and 2 (sparse) at Months 4 and 7.
In Conclusion
Based on current research, Latisse is a safe and effective treatment to enhance eyelashes and eyebrows by encouraging increased hair growth, thickness, and color.
Transitioning to a maintenance regimen of 2-3 treatments per week, after initial treatment success, also makes this treatment more affordable as a long-term solution for maintaining beauty around the eyes.
References:
1Yoelin SG et. al. A retrospective review and observational study of outcomes and safety of bimatoprost ophthalmic solution 0.03% for treating eyelash hypothrichosis. Dermatol Surg 2014;40:1118–1124.
2Zaleski-Larsen LA, Ruth NH, Fabi SG. Retrospective evaluation of topical bimatoprost and iris pigmentation change. Dermatol Surg 2017;43:1431–1433.
3 Carruthers J, et.al. Bimatoprost 0.03% for the treatment of eyebrow hypotrichosis. Dermatol Surg 2016;42:608–617.